The Central University of Technology, Free State (CUT) is committed to support government to overcome the COVID-19 pandemic. Despite this major challenge facing us as a nation, it is pleasing to report that our Centre for Rapid Prototyping and Manufacturing (CRPM), the Product Development Technology Station (PDTS), the Centre on Quality of Health and Living (CQHL), and CUT Innovation Services (CUTis) are currently involved with government in different processes related to challenges associated with COVID-19.
WATCH: Coalition of tertiary institutions making emergency ventilators, 10 April 2020
Property of eNCA
WATCH: CUT develops COVID-19 3D print masks for patients, SABC News, 02 April 2020
Property of SABC
3D Printing valves for Covid-19 patients
This project involves new concepts to assist with printing masks for non-invasive ventilation, as the hospitals have mentioned that they will not have enough ventilators. It works on the concept of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BIPAP) systems. Furthermore, anaesthetists throughout the country have requested assistance, and the Chief Executive Officers (CEOs) of some hospitals have requested assistance with possible spare parts they may need. Several old ventilators have been sourced from Universitas and Grootte Schuur hospitals, which will be used to reverse-engineer, and attempt to develop and manufacture ventilators to support hospitals nationally in this severe shortage of ventilators.
The CRPM and the PDTS at CUT are preparing for a possible spike during the next two weeks, and it can also reverse-engineer existing valves and manufacture them. Several other hospital items (such as connectors, splitters and mouthpieces/masks have been developed and manufactured by the CRPM and PDTS teams. Many of these items were manufactured through a novel approach of rapid tooling for injection moulding in appropriate polymers, which is not possible in additive manufacturing (AM), either because of the material or quantities needed.
Two major problems resulting from the outbreak have been identified, namely the lack of appropriate personal protective equipment (PPE) for hospital staff in the “hot zones”, and the need for non-invasive ventilation helmets/masks that provide patients with positive pressure and reduce the spread of the virus in the hospital.
The PDTS and CRPM are also assisting in manufacturing much-needed hospital equipment to increase the capacity of the hospitals, such as oxygen connectors and splitters.
The PDTS is a Technology Innovation Agency (TIA) Technology Station, and is funded through the Technology Station Programme (TSP) of the Department of Science and Innovation (DSI). The PDTS provides a comprehensive product development and prototyping service, boasting a world-class design, prototyping and manufacturing facility. The PDTS gained support by developing new ideas into products, or improving existing products through a detailed New Product Development Process (NPDP). The PDTS has gained valuable experience and networks to identify and develop products in South Africa, guiding projects from a basic idea, through to production and commercialisation. The PDTS provides a variety of specialised services to businesses, small- and medium-sized enterprises (SMEs) and individuals to assist throughout the product development process, or at different stages within the product development process.
Following the Medical Device Additive Manufacturing Technology Demonstrator (MedAdd) grant to the CRPM, the PDTS’ main focus shifted to the design and development of medical devices. The aim of the PDTS Medical Device Unit is not to reproduce imported medical devices, but to create novel medical devices that will solve clinical challenges within Africa. Since 2016, the PDTS has seen major growth in terms of the design and development of medical devices, growing from eight completed projects in 2016, to 51 completed projects in 2019. The PDTS is also a collaborator in the newly established MedAdd programme. The MedAdd programme aims to bridge the innovation chasm in the use of AM for the innovation, development and final manufacturing of medical devices, by enhancing the current equipment and capabilities at CUT.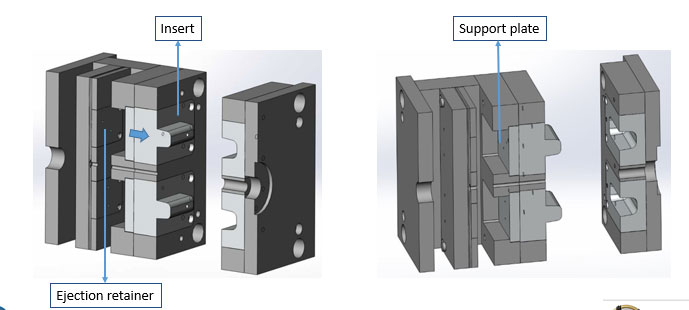
Figure 1. PDTS-developed modular injection moulding process
Over the past decade, the PDTS, supported by the Mechanical Engineering Department at CUT, has developed strong capabilities in the design and development of polymer components, as well as the production of injection moulds. The PDTS also works closely with the local injection moulding industry, creating opportunities and providing support, where possible. The PDTS has developed cost- and time-effective means of producing injection moulded parts.
Plastic injection moulded parts can be produced in as little as three days. Utilising rapid tooling inserts in a modular injection moulding system drastically reduces the capital cost and time to produce an injection moulded part. A time-effective solution for producing injection mould parts can have a major impact on the production of critical medical supplies during the COVID-19 shutdown. Figure 2 below shows an example of a rapid tooling insert produced through AM in a modular mould base.
Figure 2. Example of a rapid tooling insert produced through AM in a modular mould base
Project | Cost | Timeline |
Clinician PPE
| Need: Development cost – R80 000 Production of IM rapid tooling moulds for mask and TPU mouthpiece – R60 000 (200 units) Production of IM rapid tooling moulds for filter – R60 000 (200 units) Production of aluminium IM tooling moulds for mask and TPU mouthpiece – R120 000 Production of IM tooling moulds for filter – R120 000 1 000-unit production of masks, mouthpiece and filter housing (without filter material), at R55/unit = R55 000 Eye shield – R20/unit (R20 000) | One month – |
Non-invasive ventilation helmet | Need: Development cost – R70 000 Production of first x 20 sample run – R200 000 | One month – |
Oxygen connector ("Christmas tree connector")
| Already completed: Development cost: R10 000 Need: R80 000 (two full SLS builds) to make enough for the Free State. | Complete; ready to run production. |
Oxygen splitter connection
| Need: Development cost: R5 000 R80 000 (two full SLS builds) to make enough for the Free State. | Complete; ready to run production. |
Flutter device | Need: Production of run – R70 000 (200 units) Injection mould tooling for housing – R300 000 (*Please refer to paragraph 2.3.) | Complete; ready to run production. |
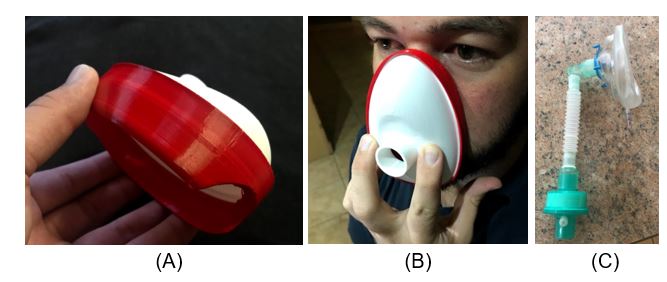
Figure 3. PPE mask: (a) prototype two of mask
The sudden impact of the virus has led to hospitals in Bloemfontein and nationally being unable to procure the needed PPE for clinicians. As of Wednesday, 25 March 2020, the PDTS has been designing and developing a reusable mask that can be manufactured through the in-house rapid tooling injection moulding process.
The masks consist of two parts, namely the main housing, and the soft interface that provides the seal. Both parts can be sterilised by autoclaving. The mask forms a tight seal around the clinician’s mouth and nose, allowing for N95 (or similar) filters to be attached, making it appropriate for the “hot zone”. “Hot zone” is the term used to describe the nearness when the clinician is working directly with the patient in close proximity, for example intubating a patient or suctioning. A filter holder has been designed that allows N95 discs to be placed in the filter housing. In extreme cases, these N95 discs can be replaced with a combination of a cotton pad and cutting a surgical mask into round discs. A clip-on face shield is attached to produce a holistic facial protection solution for the clinician.
The PDTS has produced two prototypes, tested by clinicians at Universitas hospital, Bloemfontein. A production-ready high-quality prototype design of the final injection moulding, with the exact material properties, is currently (as of 27 March 2020) being produced at the CPRM.
The prototype will be tested by the clinicians. After approval, the PDTS will produce rapid tooling inserts that will be placed in the modular injection moulds at the PDTS. The rapid tool inserts can produce 200 units. An aluminium mould insert will then be manufactured to produce +10 000 units. The aluminium inserts will take five days to manufacture. The PDTS aims to laser-cut the attachable face shields.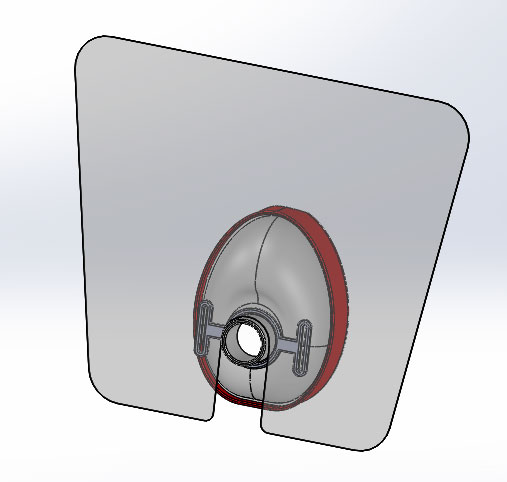
Figure 4: Proposed design of attachable face shield. (*The face shield conforms to the clinician once the straps are applied.)
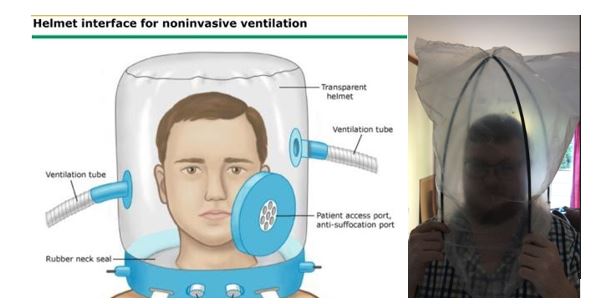
Figure 5. Concept development of non-invasive ventilation helmet
The PDTS has been working on a concept to produce a cost-effective method of producing a non-invasive ventilation helmet. This project can drastically improve the condition of patients, reducing the need for conventional ventilators, as well as provide a safer working condition for clinical professionals. The helmet/“bubble” provides positive pressure (ventilation) to the patient, and protects hospital staff from droplet spray. To ventilate the patient, a procedure called “Trans-nasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE)” will be utilised. THRIVE refers to the use of high-flow nasal cannula to augment the ability to oxygenate and ventilate a patient.
The PDTS aims to use thick plastic bags commonly used in the meat industry to form the “bubble”. Additively manufactured (SLS-nylon) flanges and a rubber/silicon/TPU neck ring will be used to seal the system. The PDTS is still busy validating the proof of concept with the clinicians, and has started the first prototype design.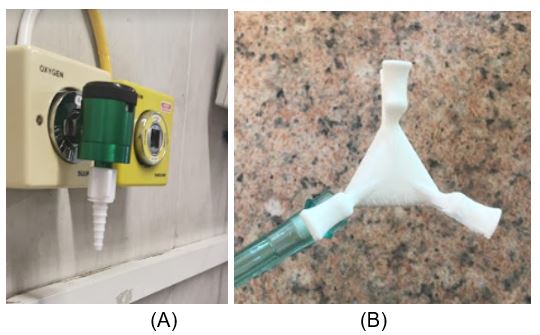
Figure 6. (A) oxygen connector; (B) oxygen splitter
Utilising the high-end EOS selective laser sintering at the CRPM, the PDTS has been producing oxygen connectors and splitters for hospitals in and around Bloemfontein.
COVID-19 patients who are admitted to hospital need to be supplied with additional oxygen. The hospitals will not have enough oxygen points to accommodate all the patients requiring oxygen.
The PDTS has produced an oxygen connector, commonly referred to in the hospitals as a “Christmas tree connector”. A design that utilises the strengths of additive manufacturing was produced. The connector allows for the supply of patient oxygen from the main supply. Additionally, a two-way splitter, with an added safety feature to ensure that the pipe does not dislodge, was produced. The oxygen splitter takes oxygen supply form one wall point, and supplies two patients with oxygen.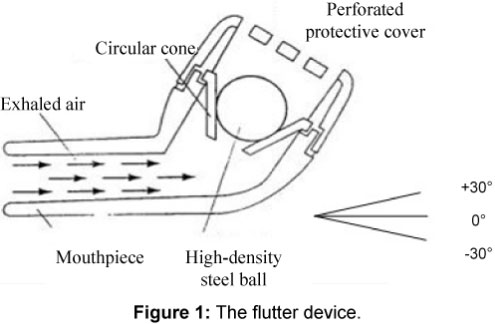
Figure 7. Current flutter devices

Figure 8. Oscillating respiratory devices (“flutter devices”)
The PDTS has developed a novel physiotherapy device to assist in removing mucus from lungs. Oscillating respiratory devices, also commonly known as “flutter devices”, currently utilise a stainless-steel ball in a cone to create vibration, as seen in Figure 7.
A flutter (oscillating air) is used to loosen mucous secretions in the pulmonary system. Physiotherapists use postural drainage positions, thus tilting patients head below feet. In this position, the current flutter will not be able to function. The PDTS has developed a plastic spring that replaces the function of the stainless-steel ball, drastically improving the functionality and user experience of the device.
A large drawback of the stainless-steel ball method is that it can only be operated at certain angles, due to its dependence on gravity to return the ball. This also implies that, to use the device, patients need to be in an upright position. The same function can be achieved utilising a plastic spring, but at any angle, and patients can also operate the device lying down. Due to the design freedom created by the plastic spring, the inlet shape can be improved. The patient is restricted from blowing air accumulated in the cheeks, only allowing air to be released directly from the lungs. Some cases of COVID-19 patients may present with a productive cough and mucus due to the destruction of pneumocystis and secondary bacterial infections. A major risk for South Africa posed by the COVID-19 pandemic is the lack of ventilators. The PDTS proposes a method of reducing the number of cases in need of a ventilator. They have completed the final design of the flutter, and manufactured the injection moulds needed for the flutter valve. Funding is needed to complete the injection mould housings. The flutter device can be rolled out into the hospitals in the current state with additive manufactured parts, but this will increase the price from R60/unit to R350/unit. To get to a unit price of R60/unit, the additional injection moulding tooling will cost R300 000.CUT is currently participating in a number of active research discussion groups, which connect virtually, namely: Medical Supplies; Rapid Manufacturing; Ventilator Development; and a COVID Agile Rapid Manufacturing Team, contributing to a team consisting of individuals from CUT, NWU, CSIR, VUT, Aerosud and Progressus Digital, to focus on an approach for urgent product development, and assessment of designs submitted by groups wanting to have a product manufactured (e.g. a valve, connector, etc.), or any new product related to COVID-19, for both design and possible manufacturing within the group. Also, liaison is taking place with the Industrial Development Corporation (IDC) to apply for support for funding (from the Department of Trade and Industry (DTI), and managed by the IDC).
Below is an extract from the COVID Team’s document, which is a living document, and new information is added on a continuous basis.

Contributions by other CUT entities
CUTis, the Centre for Applied Food Security and Biotechnology (CAFSaB), ACS Promotions and Pro Ocre are collaborating in the production of sanitisers for the Free State Department of Education. The team consists of experts within the field of Environmental Health, together with partners from business. ACS Promotions will be issuing 10 000 litres of ethanol, whilst CUTis is providing the team with the required one thousand 500-ml bottles required for packaging. The labelling will contain both CUT’s and CUTis’ logos.
Figure 9. CUT staff member with sanitiser ingredients (left); a sanitiser container (right)
Hospices will also be provided with the sanitisers, due to financial constraints experienced by these healthcare settings. On 21 March 2020, the team embarked on the pilot production of sanitisers, although this was conducted on a small scale due to a shortage of raw materials. It is, however, anticipated that there will be an ample supply and delivery of ethanol by next week, and this will enable the project to commence on a full scale. The Faculty of Health and Environmental Sciences (FHES) has also been involved in radio interviews to educate the community about COVID-19. Pamphlets and posters in both English and Sesotho are also being prepared, to make it easy for citizens to understand COVID-19.
Conclusion and contact details
The Central University of Technology, Free State (CUT)’s Rapid Research and Innovation Response to assist government with the Covid-19 pandemic, is proof of our commitment to work together in solidarity and unity to restrain the spread of COVID-19 (the coronavirus), and reduce the risk related to the community transmission phase. Therefore, we are working closely with the Department of Higher Education and Training (DHET), the National and Provincial Departments of Health, national and local agencies responsible for emergency response protocols, the National Institute for Communicable Diseases (NICD), the DSI, and industry partners, to support government with the COVID-19 pandemic.
Furthermore, a COVID-19 Research and Innovation Grant of R1 million has been made available by CUT to support these entities in their endeavours. In addition, we are also grateful for the generous support by the Department of Science and Innovation (DSI).
Enquiries:
Prof. Deon de Beer, DST Chair of ICAM: ddebeer@cut.ac.za
Dr Gerrie Booysen, Director of CRPM: gbooysen@cut.ac.za
Prof. Samson Mashele, Dean of FHES: smashele@cut.ac.z
Prof. Alfred Ngowi, DVC: Research, Innovation & Engagement, angowi@cut.ac.za